Endergonic and Exergonic Reactions
Plant and animal life maintains a high degree of organization
only because it is being resupplied with energy from the sun.
When cells convert one form of energy to another, there is a
change in the amount of potential energy. Energy changes in
living cells tend to proceed spontaneously in the direction that
results in a decrease in usable energy.
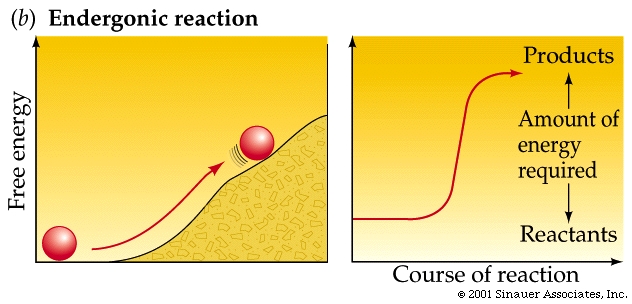
Endergonic (energy in) reactions
result in products with more energy than the reactants had.
Exergonic (energy out) reactions
result in products with less energy than the reactants had.
This animation (Audio - Important) illustrates
endergonic and exergonic reactions.
PREVIOUS
NEXT
LECTURE 19 INDEX
MAIN INDEX
